
Among several industrial failures, two that can be directly connected with tribo-corrosive degradation are (a) the 3 Mile Island Nuclear Disaster in 1979 due to a 'stuck' pressure relief valve (potentially due to adhesion) and (b) Metal on metal hip implant failures between 2007 and 2012, due to metallosis (toxicity due to excessive release of metal ions). The combination of mechanical interactions and electrochemical reactions often results in orders of magnitude higher material degradation than due to independent wear or corrosion alone. The overall material loss comprises of wear-accelerated corrosion (or chemical wear) and corrosion-accelerated wear (or mechanical wear) which varies for different materials under different corrosive environments. 'Chemical wear' is new for many tribologists and 'mechanical wear in corrosive medium is challenging to measure directly. What then are the underlying mechanisms of chemical and mechanical wear and how is this investigated ?
An accepted method is to combine electrochemical workstation with tribometers (Figure 1) in this case on a advanced modular POD 4.0. In such a configuration, the potential between WE (working electrode), typically the test sample and RE (reference electrode) is regulated and current between WE and CE (counter electrode) is measured (Figure 1) under sliding wear conditions. The counterface is made of insulating, corrosion resistant material. Corrosion current, corrosion potential, and friction can be monitored simultaneously while wear is determined by gravimetry/profilometry post-test.
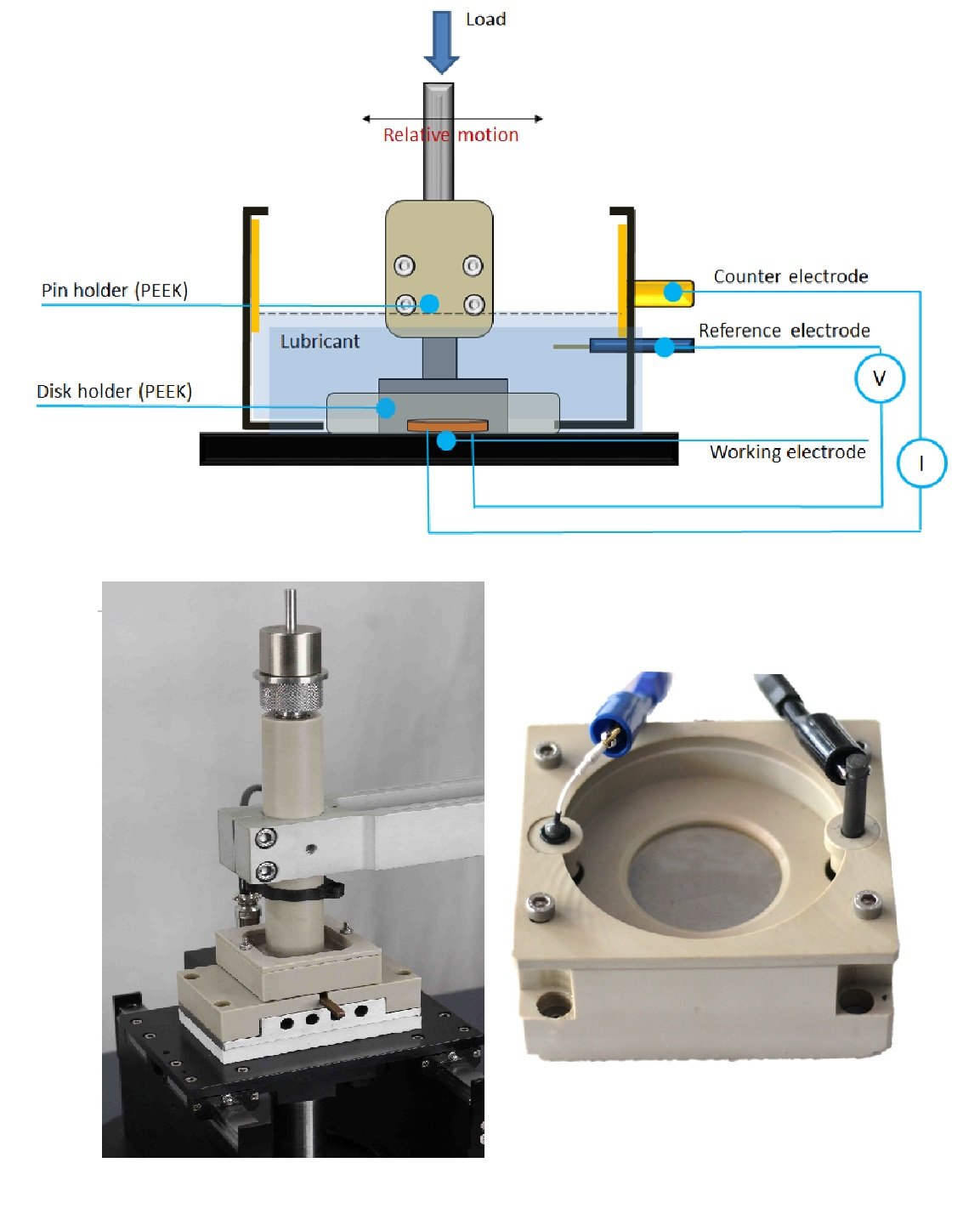 Figure 1. Schematic of Ducom POD4.0 tribometer with three electrode electrochemical cell. The actual test-set up and a close-up of the sample holder with electrode connections is shown.
Figure 1. Schematic of Ducom POD4.0 tribometer with three electrode electrochemical cell. The actual test-set up and a close-up of the sample holder with electrode connections is shown.
The total wear and dominant mechanisms (chemical vs. mechanical) can be quantified experimentally using the setup described in Figure 1 and polarization of the sample w.r.t reference electrode. The infographics in Figure 2 shows the characteristic polarization curve with an active (corrosion inhibited) and a passive regime (oxide layer formation) depending on the polarization of the sample. Measurements are conducted with no sliding, by sweeping the potential of WE from –ve to +ve potential while simultaneously measuring current, as per ASTM G59.
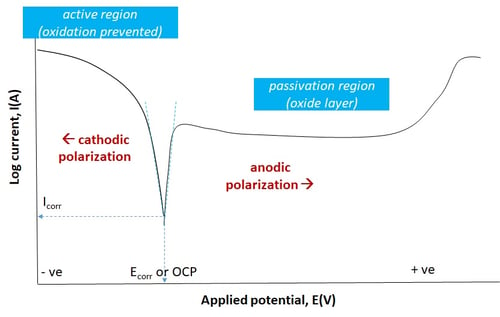 Figure 2. Characteristic corrosion response of a active-passive material showing the different regimes based on polarization potential. Such plots are used to determine the electrochemical kinetics. Note that Ecorr or OCP (open circuit potential and Icorr are the thermodynamic equilibrium values.
Figure 2. Characteristic corrosion response of a active-passive material showing the different regimes based on polarization potential. Such plots are used to determine the electrochemical kinetics. Note that Ecorr or OCP (open circuit potential and Icorr are the thermodynamic equilibrium values.
By controlling the potential of the rubbing material, the total wear and individual contributions of chemical and mechanical wear. Figure 3 depicts total degradation due to chemical wear (i.e., material loss due to dissolution as ions or oxidation due to surface de-passivation) and mechanical wear (surface oxide removal and 3rd body debris generation in corrosive medium). Of the two mechanisms, chemical wear can be quantified under anodic polarization (Figure 3).
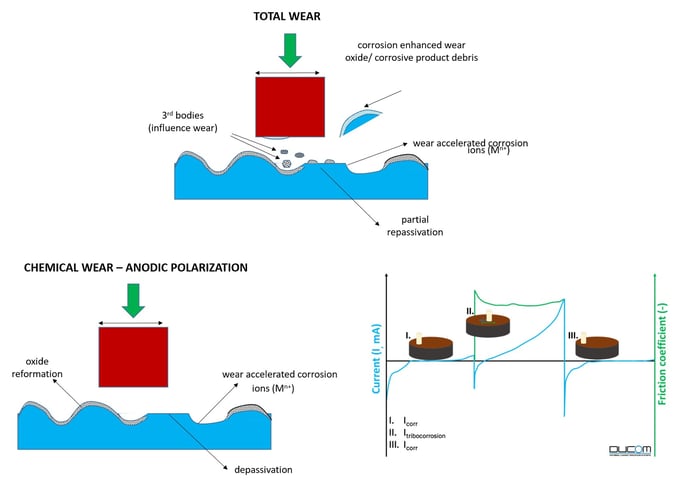 Figure 3. Overall tribo-corrosive wear mechanisms with both chemical and mechanical wear. Under anodic polarization, the chemical wear contribution can be determined from Faraday's Law of a active-passive material showing the different regimes based on polarization potential. Characteristic curves for current and friction during a tribocorrosion test under anodic polarization is shown.
Figure 3. Overall tribo-corrosive wear mechanisms with both chemical and mechanical wear. Under anodic polarization, the chemical wear contribution can be determined from Faraday's Law of a active-passive material showing the different regimes based on polarization potential. Characteristic curves for current and friction during a tribocorrosion test under anodic polarization is shown.
The test sequence has 3 steps as shown (Figure 3)
(a) stabilisation of electrochemical response and Icorr [passivation]
(b) sliding motion initiated, evolution of coefficient of friction and Itribocorr [destruction of passive layer, enlargement of wear track and enhanced corrosion]
(c) sliding motion stopped, Icorr re-established [re-passivation]
Here Itribocorr refers to the total anodic current during the sliding wear test. The excess anodic current i.e., (Iexcess = Itribocorr – Icorr, before and after sliding) is used to compute the chemical wear, Vchem, as per equation.

where M = atomic mass, Qp = passivation charge density, F = Faraday’s constant, ρ = density of metal, Iexcess = Itribocorr- Icorr = excess anodic current under anodic polarization conditions as shown in Figure 3
Usually, Itribocorr exceeds Icorr by several orders of magnitude and hence chemical wear is a matter of concern. Mechanical wear (corrosion enhanced wear) contribution is calculated by Vmech = Vtot - Vchem. as it is challenging to determine experimentally given that the electrochemical potential influences the surface properties. Such an approach for calculating mechanical wear from the measured total and chemical wear overcomes a contradiction in ASTM G119 standard. Mechanical wear Vmech should be determined under -1.0 V cathodic polarization as per ASTM G119, which is not the true corrosion enhanced wear since passivating films do not form. Moreover, hydrogen embrittlement can affect the response. The technique and measurements to determine total, chemical and mechanical wear components is summarized in Table 4.
Table 4. Electrochemical conditions and techniques to determine total, wear and individual chemical and mechanical wear contributions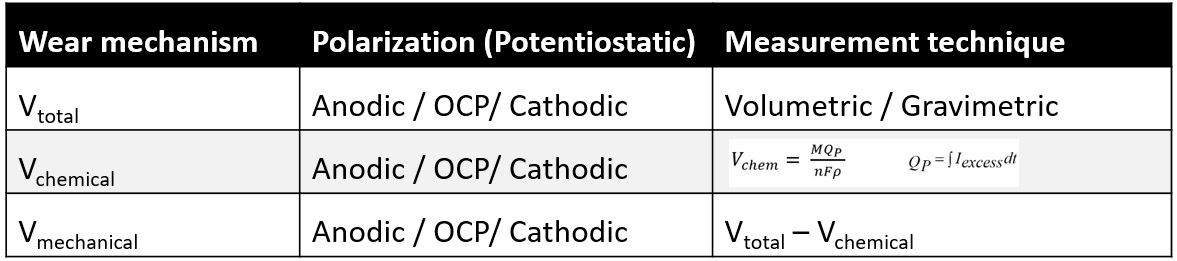
POD4.0 with tribocorrosion module complies with ASTM G99, G133, G59 and G102 standards for wear and corrosion and offers well-controlled electrochemical conditions and mechanical parameters. How has Ducom tribocorrosion enabled mechanistic understanding and deterioration of implant materials ?
Prof. Geetha Manivasagam and her research team at Vellore Insitute of Technology (India) are using Ducom Tribometer with Tribocorrosion setup for over 4 years. They have investigated the degradation of Ti-6Al-4V implants when exposed to standard body fluids (PBS and BSA) including a reactive oxygen species (ROS) such as H2O2 produced due to peri-implant inflammation. (Ref - Wear-corrosion synergistic effects on Ti6Al4V alloy in H2O2 and albumin environment, (2020), Jl. Alloys and Compounds, 830, 154539). The data from the above publication is simplified in Figures 5 and 6.
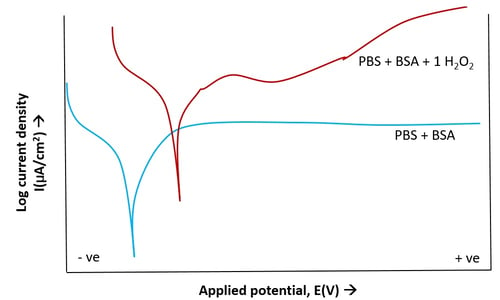 Figure 5. Potentiodynamic polarization of Ti6Al4V in (PBS+BSA) solution with and without 1% H2O2
Figure 5. Potentiodynamic polarization of Ti6Al4V in (PBS+BSA) solution with and without 1% H2O2
The polarization curves (Figure 5) shows larger current density with 1% H2O2 based on its higher oxidation potential. However, there was no abrupt change with applied voltage indicating stable oxide (TiO2) formation without breakdown.
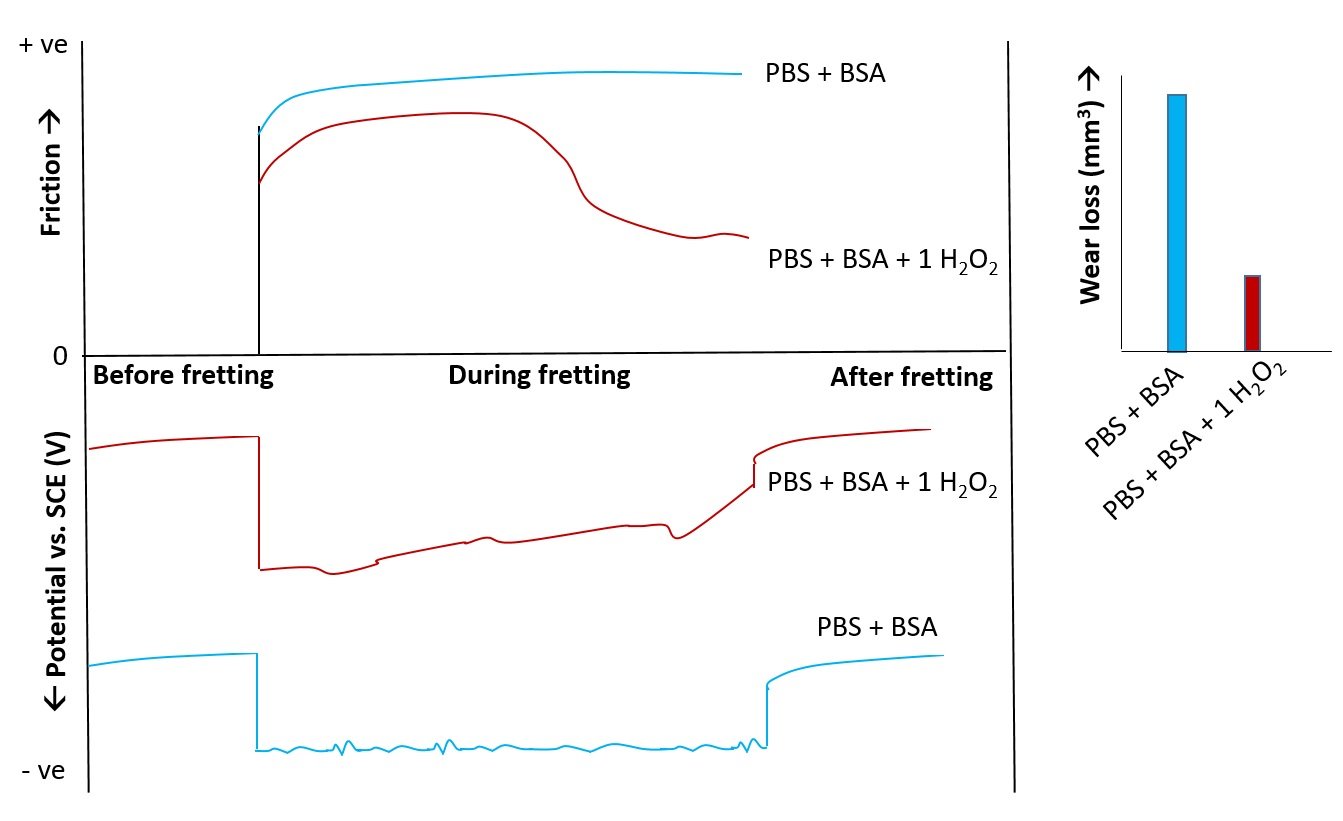
Figure 6. Evolution of friction and OCP during fretting tribo-corrosion of Ti6Al4V in (PBS+BSA) solution with and without 1% H2O2. The volumetric wear rates are plotted as bar chart.
The friction and OCP (open circuit potential) evolution were correlated and surprisingly 1 % H2O2 solution showed lower friction and wear (from profilometry) even though the polarization showed higher current density. SEM images and analysis revealed formation of Ti(IV)-H2O2 complexes over larger areas of the contact compared to formation of TiO2 films without H2O2 possibly due to the faster formation and repassivation kinetics of Ti(IV)-H2O2 complex films. The results indicate that materials with poor corrosion resistance might show superior tribo-corrosive performance under fretting corrosion, an unexpected finding.
Ducom tribocorrosion module can be integrated on our existing Universal Tribometers (Unitest and POD4) to acquire both tribological (friction, in-situ profilometry) and electrochemical (OCP, controlled polarization, corrosion current) characteristics simultaneously. Such data can enable detailed mechanistic understanding of tribo-corrosive degradation and development of materials that overcome the historical failures and last longer both inside the human body and in nuclear power plants.

These Stories on wear
USA: +1 (847) 737-1590
India: +91 (80) 4080-5555
Netherlands: +31 (85) 065 74 10